- Leukemia (cancer of the blood cells)
- Leukemia (cancer of the blood cells) can be of four types:
- Acute myeloid leukemia (AML)
- Acute lymphoblastic leukemia (ALL)
- Chronic myelogenous leukemia (CML)
Chronic lymphocytic leukemia (CLL)
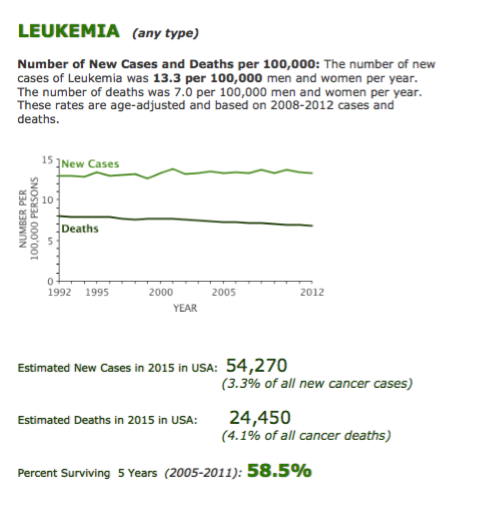
Figure 5.The most relevant epidemiological data on Leukemias, reported by the USA-SEER Agency.
Acute Leukemia, Myeloblastic (AML) or Lymphoblastic (ALL)
Acute leukemia is a type of fast growing blood cancer that requires prompt treatment. Blood is made up of different types of cells that are produced in the interior of bones in a part called the bone marrow. When people have leukemia, their bone marrow produces abnormal blood cells that are out of control and are not effectively working according to the body needs. This can cause signs and symptoms such as
- Feeling fatigues and weak
- Bleeding
- Fever and easily catching infections
The diagnosis:
Successful therapeutic plan of all blood cancers depends on the proper diagnosis at the microscopic and molecular level. This is done through comprehensive evaluation of:
- Blood tests, in particular Complete Blood Count (CBC) and blood film examination
- Bone marrow examinations, including:
- Histological evaluation
- Immunophenotypic analysis of blood and marrow cells
- Cytogenetic analysis to detect chromosomal abnormalities
- Genetic studies in order to detect disease-specific DNA abnormalities
Treatment for acute leukemia:
Treatment is basically complex and depends on the type of leukemia and other factors such as age and donor availability for bone marrow transplant.
Treatment of acute leukemia generally includes two main initial parts.
- The first part of treatment is called “induction of remission” and is approximately 4 weeks. This part of treatment requires you to stay in the hospital and receive chemotherapy regimens.
- Once in “remission” state, which means no abnormal blood cells are detected in the blood or bone marrow by microscopic examination, the second part starts, the so-called “post-remission therapy.” This part includes:
additional chemotherapy courses, for 3 to 4 months period and, in younger patients,
bone marrow transplant: either by using the patient own bone marrow cells to support the use of higher doses of chemotherapy (autologous transplant) or replacing the diseased bone marrow cells with more heathy cells from a volunteer donor (allogeneic transplant or allo-HSCT, i.e. allogeneic-Hematopoietic Stem Cell Transplantation). In fact, alloHSCT represents the most effective option for patients younger than 65-70 yrs., with Acute Leukemia and unfavorable prognostic features or with refractory or relapsed disease; the immune-mediated anti-leukemia effect, which is peculiar of allogeneic transplantation, makes the allo-HSCT procedure extremely effective in Leukemia although much more complex compared to autologous transplantation.
ü Radiation therapy is sometimes used to kill remaining leukemia cells at certain organs of the body like in cases of central nervous system involvement, usually at the end of chemotherapy courses.
The importance of disease assessment following therapy
- The accurate evaluation of response to therapy is crucial in Acute Leukemia (AL). In particular, molecular and immunophentypical procedures have been developed in order to identify minimal quantities of tumor cells that might persist following treatment below the levels detectable by standard methods of evaluation. This is called Minimal Residual Disease (MRD). The evaluation of MRD has reached a key role in the management of AL, as well as in other hematological malignancies where MRD can be assessed and monitored. In particular, in AL MRD may direct the treatment strategies following induction therapy and the preferred use of additional chemotherapy versus transplant-based is based quite often on results of MRD. Also at our Institutions at IEO, the study of MRD both in bone marrow (BM) and peripheral blood (PB) cells is a key point in the management of AL patients. In addition, research studies are ongoing with a dual aim: to optimize the use of MRD analysis in AL patients and to extend the population of patients with hematological malignancies that might be assessed and monitored by MRD.
- MRD is regularly assessed at given time points during treatment. Moreover, all patients undergo regular visits, with blood tests and additional clinical exams, if needed, during the various treatment steps and at long term once the whole treatment program is completed
Within our OM-2 Division at IEO, there are sub-unit of doctors with specific interest in each of the main hematological malignancies, i.e. Acute Leukemia with the alloHSCT program, Lymphoma and Multiple Myeloma. The doctors mainly involved in the management of Acute Leukemia and in the allogeneic transplant procedures are: Dr. Rocco Pastano (Director of the allogeneic-HSCT program), Dr. Federica Gigli and Dr. Simona Sammassimo along with the support of Dr. Safaa Ramadan
Chronic leukemia
Chronic Lymphocytic Leukemia (CLL), and Chronic Myeloid Leukemia (CML)
Chronic leukemia is a type of blood cancer that usually grows slowly.
Main signs and symptoms are:
- Feeling fatigues and weak
- Easily catching infections
- Fevers, drenching sweats at night, and losing weight
- Lymphadenopathy and organomegally: patients with Chronic Lymphocytic Leukemia (CLL) may have swollen lymph nodes in the neck, under the arm, or in the groin; patients with Chronic Myeloid Leukemia (CML) may present with large spleen.
The diagnosis:
- CBC (Complete Blood Count), along with blood film analysis, is the first step to suspect a Chronic Leukemia
- The diagnosis of CLL can be made by Immunophenotypic Analysis, that may show the presence of the a clonal Lymphocyte population, i.e. the neoplastic CLL population
- CML diagnosis requires the detection by molecular analysis of blood or marrow DNA, revealing the presence of the distinctive bcr-abl gene, that originate by the translocation involving chromosomes 9 and 22, giving rise to the diagnostic “Philadelphia Chromosome” (Ph-1)
Treatment of chronic leukemia:
Chronic lymphocytic leukemia (CLL):
Being slowly growing and indolent tumor, CLL is treated when the disease causes symptoms or starts growing faster than its original rate.
The main form of treatment is chemotherapy combined with immunotherapy. The choice between different chemotherapy drugs is based not only on the molecular profile of the disease but also on patient related factors and comorbidities. Immunotherapy is mainly represented by monoclonal antibodies directed against surface antigens of neoplastic Lymphocyte. By far, the most extensively employed is the anti-CD20 monoclonal antibody Rituximab. Other monoclonal antibodies and novel drugs directed against molecular checkpoints that are abnormally expressed in CLL cells are under advanced development (see also: Lymphoma section, Novel targeted drugs)
Chronic myeloid leukemia (CML):
CML cells harbor the abnormal bcr-abl gene, whose product is a tyrosine-kinase responsible for the over-production of myeloid blood cells. In the last two decades, very effective drugs inhibiting the bcr-abl tyrosine-kinase product (known as Tyrosine-kinase inhibitors or TKIs) have been developed, with a of great consequence for the management and the long-term life-expectancy for patients with CML. Nowadays, most CML patients are successfully treated with TKIs, without need of neither chemotherapy nor transplant procedures . TKIs are very effective in long term control of the disease and resulted in a revolutionary success in the history of this disease. Treatment requires frequent visits to evaluate the course of the disease response with frequent blood and as necessary bone marrow exams.
In few cases, the cancer blood cells grows faster and becomes less controlled by the “first generation” TKI; however, newly developed, “second or third generation” TKIs, may overcome drug-resistance.
Other treatment options, which can help to control the usually infrequent advanced stages of the disease are:
- Chemotherapy: a number of regimens can be available.
- Bone marrow transplant: after intensive chemotherapy, a healthy donor bone marrow cells are used to replaces cells in the bone marrow of the patient.
Staff
The doctors mainly involved in the management of Acute Leukemia and in the allogeneic transplant procedures are: Dr. Rocco Pastano (Director of the allogeneic-HSCT program), Dr. Federica Gigli and Dr. Simona Sammassimo along with the support of Dr. Safaa Ramadan